|
|
|
The effect of microplastics on the feeding activity of the ascidian Pyura stolonifera
|
|
Chantel Ulani Say 2017
|
|
|
|
Abstract | |
Vast quantities of plastics are polluting our oceans. However, the consequences that these plastics may have on marine suspension feeders, such as the ascidian Pyura stolonifera, are not well understood. The ingestion of inorganic particles (≥40μm) causes blockages within the branchial basket of P. stolonifera, which is likely to impede their ability to feed (Armsworthy 1997; Macginitie 1939). Therefore, this study aims to extend upon these findings by determining if an ascidian’s ability to feed is impeded by the ingestion of 40μm microplastics. Pyura stolonifera were force-fed four concentrations of microplastics (n=4/ treatment), and the amount of algae present within the water (optical density at 550nm) was recorded every 30 minutes, for two hours, starting at time zero. Treatment (the concentrations of microplatics) had a significant effect on the feeding activity of the ascidian, P. stolonifera. Time also significantly affected their feeding activity, with the optical density readings increasing over time. During this experiment, P. stolonifera were observed to squirt out water, possibly to expel the microplastics (and in turn any algae that was consumed) potentially causing this increase in algal density with time. There are two possible triggers for this squirting behaviour; ascidians can discriminate particle selection based on (1) their size (and density) and/or (2) the chemical composition of the particles (Bingham & Walters 1989; Klumpp 1984; Young 1988). Therefore, future studies are necessary to elucidate whether P. stolonifera are indeed able to detect and discriminate between organic and inorganic particles, as this may explain the result of this study.
|
|
|
Introduction | |
In recent years there has been a growing concern about the sheer volume of plastic pollutants within our oceans, which is predicted to continue to increase in the future (Anderson, Park & Palace 2016; Auta, Emenike, & Fauziah 2017). Worldwide there has been an increase from 5 million tons of plastic production to 322 million in 2015 (Gewert et al. 2017); where it is predicted that there is an order of 250,000 tons of plastics currently floating within our oceans (Enders et al. 2015). The major contributor to plastic pollutants is litter from land-based sources, which is usually classified into two distinct groups: macroplastics (particle exceeding 5mm in diameter) and microplastics (particles less than 5mm in diameter) (Gewert et al. 2017). Microplastics can further be defined as either primary microplastics - manufactured to be that small; commonly used in cosmetics, toiletries and clothing, and secondary microplastics - those formed from fragmentation and degradation of larger pieces of plastic debris (Auta, Emenike, & Fauziah 2017; Gewert et al. 2017).
 Figure 2: Densely packed Pyrua stolonifera found within the costal rocky intertidal zone of Shelly Beach, Caloundra, Australia. Photographs: Chantel Say Figure 2: Densely packed Pyrua stolonifera found within the costal rocky intertidal zone of Shelly Beach, Caloundra, Australia. Photographs: Chantel Say
Microplastics have the potential to impose detrimental effects on the health of a wide range of taxa (Auta, Emenike, & Fauziah 2017) as they impede the ability of an individual to feed and digest food, increasing stress and reducing the fecundity and survival of the individual (Cole et al. 2015). Marine organisms have the potential to not only consume microplastics directly from the water column but also indirectly, as microplastics can also be transferred up through the food chain (Cole et al. 2015); a study conducted by Cole et al. (2015) found the presence of microplastics within the gut of copepods, a food source of ascidians. Ascidians are highly efficient suspension feeders that play an important role in marine benthic communities (Figure 1; Jaffar Ali & Tamilselvi 2016). In fact, ascidians are key stone species found to be extremely dominant and widespread throughout many habitats, including areas with high human impact (Figure 2; Jaffar Ali & Tamilselvi 2016). Therefore, previous studies have identified ascidians as an ideal candidate for a bio-indicator to monitor the impact of pollution in marine ecosystems (Bellante et al. 2016).
Ascidians are sessile macro-invertebrates commonly known as tunicates because of their tough protective test, the tunic, which encases the soft animal inside (Figure 3, 4 and 5; Armsworthy 1997). They can be either solitary, a single zooid within the tunic (e.g. P. stolonifera) or colonial, containing multiple zooids within a single tunic. Ascidians filter water through two siphons located on the anterior end - a bracnhial (inhalant) siphon and an atrial (exhalant) siphon (Figure 6; Armsworthy 1997). The inhalant siphon leads to a large net-like structure known as the branchial basket, an organ that is vital for collecting food and the uptake of oxygen (Figure 7; Armsworthy 1997). Water is drawn in through the inhalant siphon by the beating of lateral cilia, which line the gill slits (the stigmata openings) of the branchial basket (Figures 7 and 8; Armsworthy 1997; Petersen & Svane 2002; Pestarino, Fiala‐Medioni & Ravera 1988).
 Figure 3: The ascidian, Pyura stolonifera, found within the costal rocky intertidal zone of Shelly Beach, Caloundra, Australia. Photograph: Chantel Say Figure 3: The ascidian, Pyura stolonifera, found within the costal rocky intertidal zone of Shelly Beach, Caloundra, Australia. Photograph: Chantel Say
This allows the ascidian to suspension feed, removing particulate matter (commonly plankton and detritus) from the water column (Armsworthy 1997). Water passes through the branchial basket into the atrial cavity where it is then expelled through the exhalant siphon. Suspended particles within the water are trapped within the mucus sheet (secreted by the endostyle) that lines the surface of the basket as the water passes through it (Figure 8; Armsworthy 1997; Petersen & Svane 2002; Pestarino, Fiala‐Medioni & Ravera 1988). These particles are entangled with the mucus and then rolled into a string/cord (Petersen & Svane 2002), which is drawn down through the pharynx and the esophagus by frontal cilia into the stomach, the location of digestion (Armsworthy 1997).
 Figure 6: The ascidian, Pyrua stolonifera, found within the costal rocky intertidal zone of Shelly Beach, Caloundra, Australia. Inhalant and exhalant siphon labeled. Photograph: Chantel Say Figure 6: The ascidian, Pyrua stolonifera, found within the costal rocky intertidal zone of Shelly Beach, Caloundra, Australia. Inhalant and exhalant siphon labeled. Photograph: Chantel Say
 Figure 8: Simplified diagram of the water current and particle movement through an ascidian when feeding. As water enters the inhalant siphon and travels through the branchial basket, a sticky mucus sheet lining the basket traps particles suspended within the water. These particles are then transported by cilia down through the esophagus to the stomach where digestion occurs. Waste products will then travel through the intestine, to the anus where it will be expelled through thee exhalant siphon along with the remaining water, which travels through the branchial basket. Photograph: Chantel Say Figure 8: Simplified diagram of the water current and particle movement through an ascidian when feeding. As water enters the inhalant siphon and travels through the branchial basket, a sticky mucus sheet lining the basket traps particles suspended within the water. These particles are then transported by cilia down through the esophagus to the stomach where digestion occurs. Waste products will then travel through the intestine, to the anus where it will be expelled through thee exhalant siphon along with the remaining water, which travels through the branchial basket. Photograph: Chantel Say
The amount of food an ascidian consumes is dependent on the type and quantity of particles it encounters. As ascidians are sessile suspension feeders, they are highly vulnerable to being exposed to unfavourable conditions such as high concentrations of inorganic material (i.e. suspended sediment) from tidal fluctuations and storms (Armsworthy 1997). However, it is still not confirmed whether ascidians possess the ability to discriminate between organic and inorganic particles. The consumption of inorganic particles can have many negative effects on ascidian health. Firstly, inorganic particles do not contain vital nutrients. Secondly, ascidians have been found to stop feeding upon filling their gut capacity (Petersen & Riisgård 1992; Petersen, Mayer & Knudsen 1999). Therefore, as inorganic particles cannot be digested, it is possible that the ascidians feeding ability may be restricted altogether. Thirdly, particles greater than 40μm can result in blockages of the branchial cavity; restricting access to required food and oxygen (Armsworthy 1997; Macginitie 1939). Finally, inorganic particles found in the ocean like microplastics have the potential to release toxins, potentially impeding their ability to feed (Cole et al. 2013). Therefore the consumption of suspended particles, in particular inorganic particles, has the potential to cause starvation, thus impeding the growth, development and survival of the ascidian P. stolonifera (Cole et al. 2015). Thus the present study aims to gain a better understanding of how ingested inorganic particles (microplastics) affect the feeding activity of the ascidian P. stolonifera.
|
 |
| Figure 1 |
|
 |
| Figure 2 |
|
 |
| Figure 3 |
|
 |
| Figure 4 |
|
 |
| Figure 5 |
|
 |
| Figure 6 |
|
 |
| Figure 7 |
|
 |
| Figure 8 |
|
|
|
Materials and Methods |
Collection of biological material | |
The ascidians, Pyrua stolonifera, were collected from the coastal rocky intertidal shore of Shelly Beach, Caloundra in May 2017 (Figure 9 and 10). After being removed from the rocky substratum, the ascidians were transported to an aquarium at the University of Queensland (UQ), that same day, where they remained prior to experimentation. All ascidians used in this experiment (n=16) were approximately the same size (ranging from 15cm3 to 190cm3).

Figure 9: The costal rocky intertidal zone of Shelly Beach, Caloundra, Australia; the location site where the ascidians for this study were collected. Photograph: Chantel Say

Figure 10: The ascidian, Pyura stolonifera, found within the costal rocky intertidal zone of Shelly Beach, Caloundra, Australia. Photographs: Chantel Say
|
 |
| Figure 9 |
|
 |
| Figure 10 |
|
|
|
Experimental approach | |
To assess how microplastics affect the feeding behaviour of the ascidian, P. stolonifera, three different concentrations of 40μm microbeads (polymethyl methacrylate - Spheromers) were added to artificial seawater. The three treatments corresponded to a low, medium and high concentration of microplastics consisting of 0.05g/ml, 0.1g/ml and 0.15g/ml, respectively. An artificial seawater control (no microbeads) was also included.
Sixty-minutes prior to the start of the experiment, ascidians were placed into individual experimental tanks (approximate size 785cm3) containing (400ml) of artificial seawater. A 0.25ml liquid algae mix was added to each experimental set up, as the pilot study demonstrated that the ascidians exhibited normal feeding behavior (open siphons) at this concentration (data not shown). The algae mix included four different algae types that varied in size and average cell count/ml: Nannochloropsis sp (1-2 microns, 68 billion/ml), Pavlova sp (4-7 microns, 3.3 billion/ml), Isochrysis (5-6 microns, 4 billion/ml), and Thalassiosira weissflogii (7-20microns, 0.32 billion/ml). All four types of algae were used in this algal solution as it is unknown which type/size of algae is a preferred food source of ascidians. For each treatment, a volume of 0.5ml was slowly pipetted directly into the inhalant siphon of each individual (n=4/ treatment; Figure 9) including the control treatment, which contained only seawater (no microbeads). Note the net average size of all the ascidians for each treatment was approximately 50cm3 (±10cm3).
|
|
|
Data collection | |
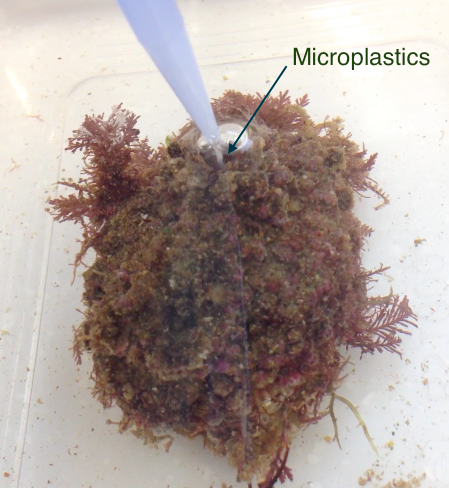 Figure 11: Force-feeding the ascidian, Pyura stolonifera, by pipetting the microplastic treatment directly into the inhalant siphon (experimental method to determine if microplastics influence the ascidians feeding activity). Note you can see a stream of microplastics flowing directly into the siphon. Photograph: Chantel Say
P. stolonifera were force-fed four concentrations of microplastics (n=4/ treatment) by pipetting the treatments directly into the inhalant syphon (Figure 11). The amount of algae present within the water (optical density) was recorded every 30 minutes, for two hours, starting at time zero; stirring water gently before samples taken to insure algae was suspended. Note the pipetting for the each ascidian was staggered in order to make this possible. Figure 11: Force-feeding the ascidian, Pyura stolonifera, by pipetting the microplastic treatment directly into the inhalant siphon (experimental method to determine if microplastics influence the ascidians feeding activity). Note you can see a stream of microplastics flowing directly into the siphon. Photograph: Chantel Say
P. stolonifera were force-fed four concentrations of microplastics (n=4/ treatment) by pipetting the treatments directly into the inhalant syphon (Figure 11). The amount of algae present within the water (optical density) was recorded every 30 minutes, for two hours, starting at time zero; stirring water gently before samples taken to insure algae was suspended. Note the pipetting for the each ascidian was staggered in order to make this possible.
Optical density was used to measure the concentration/density of algal cells within the water. Optical density is determined by the amount of light which is able to pass through a medium; a high value (absorption) means that low amounts of light could pass through and therefore there is a high number of cells and vice versa (Griffiths et al. 2011). In order to determine the amount of algae present (cell density), each water sample was analysed using a general purpose UV/Vis Spectrophotometer (DU 720) at a wavelength of 550nm. The pigment content of microalgae is variable (i.e. each can vary in the amount of light they absorb) (Griffiths et al. 2011). Therefore, by using a wavelength that is beyond the absorbance range of these pigments (i.e. 550nm - the minimum absorption for chlorophyll) only the cell density (and not pigment content) will be recorded, minimizing error and thus increasing the accuracy of the results (Griffiths et al. 2011).
|
 |
| Figure 11 |
|
|
|
Data analysis | |
These data were analysed using the statistical analysis program R-3.3.0. An analysis of co-variance (ANCOVA) was used to test for a significant correlation between the effect of treatment (four different concentrations of microplastics) and time, on the feeding activity of the ascidian Pyura stolonifera as measured by algal density in the water.
|
|
|
|
Results |
Results of main experiment | |
Time had a significant effect on the optical density measurements (ANCOVA, n=4/treatment: F 1, 4 = 3.482, P = 0.0127) as at 120 minutes the algal concentration was significantly different from time zero (t-test: P=0.0031). There was also a significant interaction between treatment (the concentration of microplastics) and the time P. stolonifera spent within the water (ANCOVA, n=4/treatment: treatment x time F 1, 12 = 2.062, P = 0.0337), whereby the effect of time on algae abundance was not the same for all algal concentrations (Figure 12).
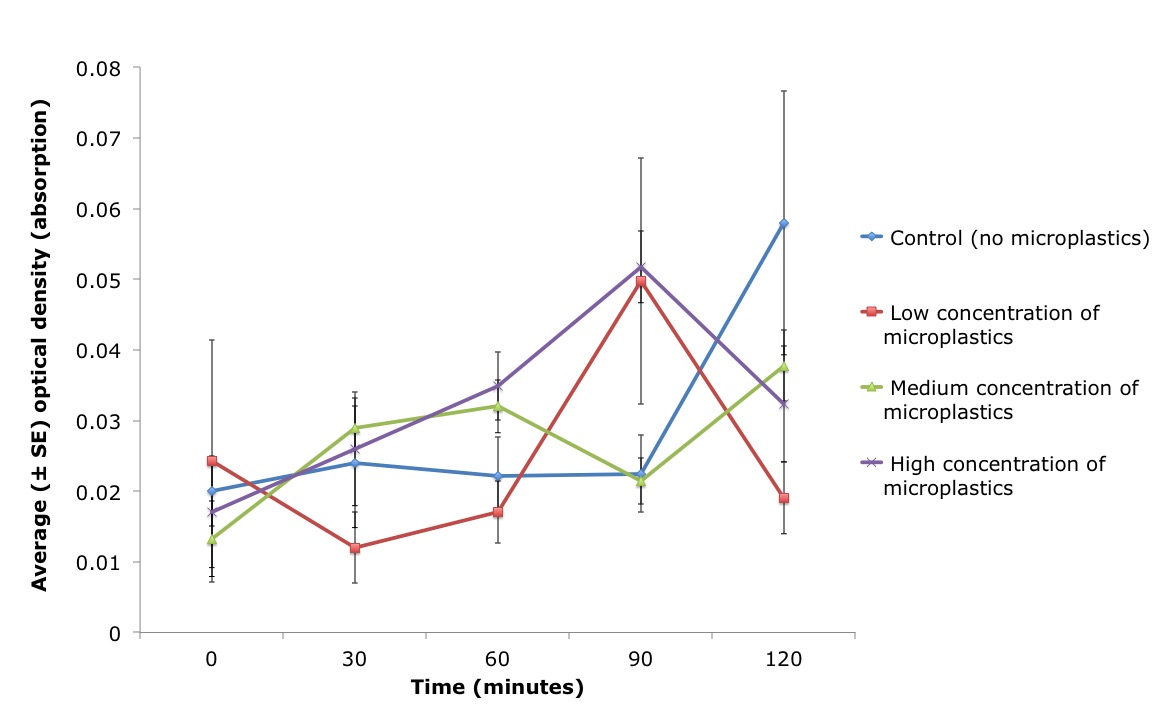 Figure 12: Average optical density (absorption) of algae cells present within artificial water (± standard error (SE)) over time (minutes), for three different treatments and one artificial seawater control (blue line). Three concentrations of microbeads were pipetted into the inhalant siphon of the ascidian, Pyura stolonifera (n=4/treatment) to test for an effect on feeding activity. The low (red line), medium (green line) and high (purple line) concentration of microplastics used in this study corresponded to 0.05g/ml, 0.1g/ml and 0.15g/ml, respectively. Water samples were taken every 30 minutes (including time zero) and optical density was measured. There was a significant correlation between the density of algae cells present (optical density) in the water and the concentration of microplastics pipetted directly into the ascidian (ANCOVA, n=4/treatment: treatment x time F 1, 12 = 2.062, P = 0.0337). Figure 12: Average optical density (absorption) of algae cells present within artificial water (± standard error (SE)) over time (minutes), for three different treatments and one artificial seawater control (blue line). Three concentrations of microbeads were pipetted into the inhalant siphon of the ascidian, Pyura stolonifera (n=4/treatment) to test for an effect on feeding activity. The low (red line), medium (green line) and high (purple line) concentration of microplastics used in this study corresponded to 0.05g/ml, 0.1g/ml and 0.15g/ml, respectively. Water samples were taken every 30 minutes (including time zero) and optical density was measured. There was a significant correlation between the density of algae cells present (optical density) in the water and the concentration of microplastics pipetted directly into the ascidian (ANCOVA, n=4/treatment: treatment x time F 1, 12 = 2.062, P = 0.0337). |
 |
| Figure 12 |
|
|
|
Results of validation experiment | |
A wavelength of 550nm was chosen to detect algal cell density, as conducted in Griffiths et al. (2011). However, this validation experiment demonstrated that a wavelength of 550nm detected both algal cells and microplastics (Figure 13). There was a significant difference in the optical density between the algae only treatment and a mix of both algae and microbeads (ANOVA, n=3/treatment: F 1, 2 = 482.8, P = 1.845e-7). The microplastic only treatment also gave a reading of 0.2224 indicating that the microbeads were indeed being detected at a wavelength of 550nm.
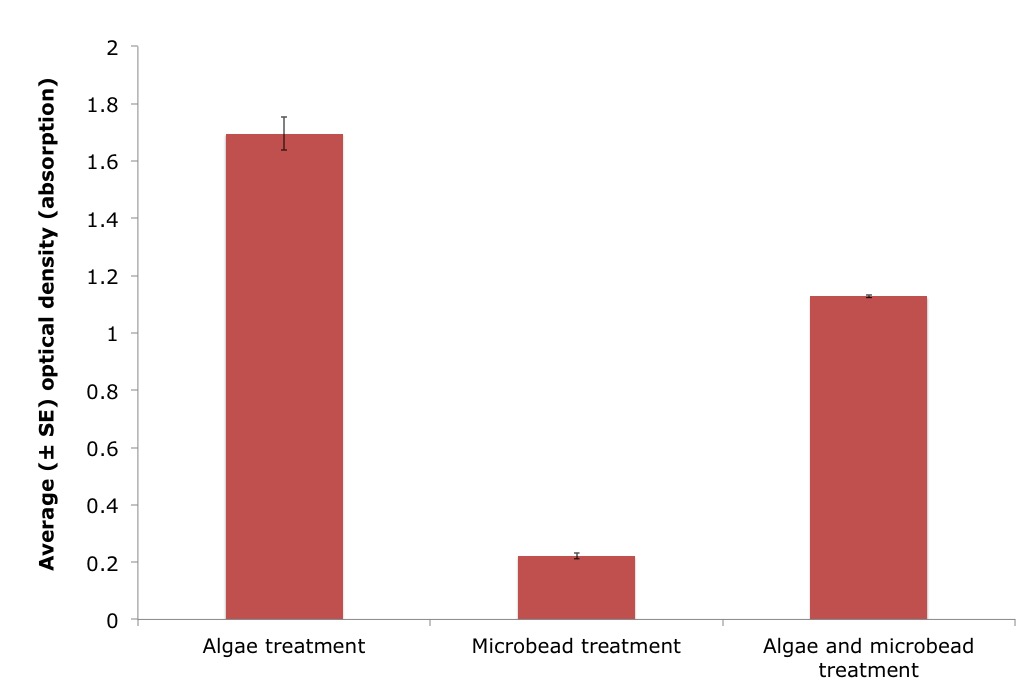
Figure 13: Average optical density (absorption), using a wavelength of 550nm, for three different treatments including algae, microbeads and a mix of both algae and microbeads, in artificial seawater (± standard error (SE)). Treatment significantly effected the optical density measurements (ANOVA, n=3/treatment: F 1, 2 = 482.8, P = 1.845e-7). |
 |
| Figure 13 |
|
|
|
|
Discussion |
The behaviour of Pyura stolonifera is affected by high concentrations of suspended particles | |
Previous studies have demonstrated an inverse relationship between the concentration of inorganic particles (silt) and feeding (Klumpp 1984). Despite inorganic particles, sized 0.5-20μm, having no direct effect on the feeding activity of P. stolonifera (maintaining a 100% efficiency rate of particle consumption; Klumpp 1984); the ascidians likely do not meet their nutritional requirements. Additionally, ascidians are unable to digest inorganic particles. Therefore a gut full of these particles could have downstream consequences on the health of the individual. Particles > 40μm clog the stigmata (creating blockages of the mucus net) of the P. stolonifera branchial basket, restricting feeding (Armsworthy 1997; Macginitie 1939). Therefore, as 40μm microbeads were used in this study, it was expected that the algae remaining in the water would be greatest for the ascidians exposed to the highest microplastic concentration (i.e. they consume the least amount of algae). However, this study illustrated an overall increase in the amount of algae present within the water, where the control was seen to have the most algae at 120 minutes (Figure 12).
It is possible that the observed increase in optical density was a result of technical error. For example, if the cuvettes were not completely clean, this could have affected the results. The concentration of algae used for this experiment was also chosen based on a pilot study that assessed feeding behaviour of the ascidians (open siphons). However, it is possible that the spectrophotometer was not sensitive enough to detect changes in algae concentration, and this could thus be a potential limitation of this study.
Alternatively, there is a biological explanation for the results of this study. These ascidians may have the ability to reject the microbeads (using size and/or chemical signals of the particles). The rejection of microbeads by the ascidians could thus explain the increase in optical density over time, if these microbeads were detected by the spectrophotometer at a wavelength of 550mn. A validation experiment confirmed that the microplastics were indeed detected at a wavelength of 550nm (see Figure 13 for the results of the validation experiment).
Suspended particles within the water column, whether organic or inorganic, can negatively impact filtration rate (a proxy for the feeding activity) of an ascidian (Petersen, Mayer & Knudsen 1999), either directly through the control of ciliary beating or indirectly by increasing the behavior of squirting (Petersen & Riisgård 1992). Ascidians alter the beat frequency of their lateral cilia in response to their gut capacity and digestive system (Petersen & Riisgård 1992; Petersen, Mayer & Knudsen 1999). Ascidians reduce cilia beating, and as a result reduces filtration rate, by 50% in response to exposure to high concentrations of algae (8,000 to 20,000 cells ml-1) (Petersen & Riisgård 1992; Petersen, Mayer & Knudsen 1999). There are two possible explanations for this behaviour. Firstly, ascidians may adopt this strategy to prevent over-loading of the gut and secondly, the rate of consumption by the ascidian is potentially constrained by the efficiency of the digestive enzymes present in the gut; thus seen more as a regulatory mechanism (Petersen, Mayer & Knudsen 1999; Petersen & Riisgård 1992). The reduction in cilia beating, and non-efficient feeding, may explain why the algal concentrations did not decrease, over time in this study, as would be expected if ascidians were consuming algae (Figure 12). Therefore, these previous studies support the hypothesis that these ascidians were able to detect and reject to these microplastics, explaining the increase in optical density over time.
|
|
|
Distinguishing between particles based on size | |
Some suspension feeders like the bivalve molluscs, have the ability to distinguish between organic and inorganic particles, allowing the rejection of particles with low nutritional value (Armsworthy 1997). However, it is unclear whether ascidians also possess the ability to sort organic particles from inorganic particles. Petersen, Mayer & Knudsen (1999) suggested that ascidians are only capable of preventing consumption of unwanted particles by altering their ciliary action (i.e. reducing frequency rate), closing their inhalant syphon or squirting water out of their siphon. Squirting water out of their siphon is a common behaviour for ascidians associated with aerial exposure, changes in temperature and the expulsion of fecal waste and gametes. This behaviour is accomplished by the contraction of the body wall ejecting water within the pharyngeal and the atrial cavity out through the exhalant siphon (Armsworthy 1997; Petersen, Mayer & Knudsen 1999).
 Figure 14: The Pyrua stolonifera performing the behaviour of squirting (illustrated by the plume of microplastics) after a concentration of microplastics were pipetted directly into the inhalant syphon. Photograph: Chantel Say
In contrast, some previous studies have hypothesised that ascidians are capable of selecting food based on size, as Klumpp (1984) observed both sand and phytoplankton in the branchial cavity, but only phytoplankton cells had been passed to the gut. The oral tentacles are one potential mechanism for discriminating between particles of varying sizes. When foreign particles come into contact with these tentacles, this triggers the squirting behaviour (Armsworthy 1997; Macginitie 1939). In P. stolonifera, this squirting behavior has been associated with high particle density and the presence of particles ≥40μm (Armsworthy 1997; Klumpp 1984). Indeed, in this study P. stolonifera demonstrated this squirting behaviour as well (Figure 14). Therefore, this detection and response to different sized microplastics may explain why the algal concentrations did not decrease over time in this study (Figure 12), as the concentrations (and method of injection) of these microbeads were much higher than would be expected in a natural environment.
Figure 14: The Pyrua stolonifera performing the behaviour of squirting (illustrated by the plume of microplastics) after a concentration of microplastics were pipetted directly into the inhalant syphon. Photograph: Chantel Say
In contrast, some previous studies have hypothesised that ascidians are capable of selecting food based on size, as Klumpp (1984) observed both sand and phytoplankton in the branchial cavity, but only phytoplankton cells had been passed to the gut. The oral tentacles are one potential mechanism for discriminating between particles of varying sizes. When foreign particles come into contact with these tentacles, this triggers the squirting behaviour (Armsworthy 1997; Macginitie 1939). In P. stolonifera, this squirting behavior has been associated with high particle density and the presence of particles ≥40μm (Armsworthy 1997; Klumpp 1984). Indeed, in this study P. stolonifera demonstrated this squirting behaviour as well (Figure 14). Therefore, this detection and response to different sized microplastics may explain why the algal concentrations did not decrease over time in this study (Figure 12), as the concentrations (and method of injection) of these microbeads were much higher than would be expected in a natural environment.
Alternatively, the injection of the liquid directly into the inhalant siphon may have induced the observed squirting behaviour. This explains why the controls (which were injected with just artificial seawater) demonstrated this squirting behaviour as well as the three microplastic treatments (Figure 12). Even though the treatments were pipetted into the ascidian slowly, in an attempt to prevent rejection, the flow rate would have been greater than the natural current created by their cilia. This may have shocked the individual (possibly resulting in stress) whereby the individual reacts by expelling the water.
Furthermore, the ascidians may have expelled any algae that were consumed prior to the pipetting. Each ascidian was left siting in water containing 0.25ml of liquid algae mix for at least an hour prior to the experiment, to acclimatize. A pilot study indicated some time is required for the ascidian to relax before normal feeding (open siphons) is observed in high concentrations of algae. Therefore, it is thus possible that by disturbing the ascidians this triggered squirting which intern could have resulted in the ascidian to expel any previously consumed algae. This can also explain why the amount of algae concentrations unexpectedly increased over time (Figure 12).
|
 |
| Figure 14 |
|
|
|
Distinguishing between particles based on chemical signals | |
Another possible trigger for this squirting behavior is the detection of chemicals. Studies have suggested that ascidians possess a mechanism to distinguish between particles based on their chemical composition (Bingham & Walters 1989; Young 1988). A study conducted by Bingham & Walters (1989) demonstrated that three species of ascidians rejected eggs and larvae that were present in the water column. In addition, a similar study found selection for and against sperm in cannibalistic or non-cannibalistic ascidians, respectively (Young 1988). Non-cannibalistic ascidians strongly reject sperm and cannibalistic ascidians accepted sperm 50% of the time (Young 1988). Both these studies suggest that ascidians are able to respond to and discriminate between particulate matters suspended in the water based on chemical signals (Bingham & Walters 1989; Young 1988).
Microplastics have the potential to be a vector introducing chemical and toxins into the marine ecosystem. During manufacturing, added properties (such as phthalates and polybrominated diphenyl ethers) are incorporated into microplastics (Cole et al. 2013). Thus these toxicants have the potential to leach out of the plastic upon weathering (Cole et al. 2013). Thus its possible that ascidians are able to detect these unnatural chemicals within the microplastics used within this study and hence reject them. Additionally, Seiderer & Newell (1988) suggest that P. stolonifera is capable of selecting algae based on its composition. If ascidians are indeed capable of determining the composition of the particles, ascidians may reject microplastics on the premise that they can detect toxic chemical and/or lack the ability to digest them. In both cases the ascidian would reject the microplastics and hence trigger the squirting behaviour impeding their potential feeding time. A future study could thus investigate whether microplastics release toxins, and if they do, whether the ascidian, in particular P. stolonifera, have the ability to detect these and if this triggers the squirting behaviour that was observed in the present study.
|
|
|
Concluding remarks | |
The presence of microplastics in our oceans could have huge ecological implications, affecting the health of marine invertebrates such as the ascidian P. stolonifera. The present study demonstrated that microplastics, which were injected into the inhalant siphon, might have caused a squirting behaviour in P. stolonifera. This behaviour could explain why the amount of algae present within the water significantly increased over time. However, to eliminate the possibility that this squirting behaviour was merely a response to the “injection method”, future research is necessary in order to confirm that the ascidian Pyura stolonifera can indeed detect and respond to microplastics (based on their size and/or chemical composition) and consequently expel the water containing these particles.
|
|
|
|
Acknowledgements | |
Thank you to Professor Bernard Degnan, Assoc/Professor Sandie Degnan and the tutors for their scientific guidance; and to the School of Biological Science at the University of Queensland, for the use of the laboratory and aquarium facilities along with equipment.
|
|
|
References | |
Anderson, JC, Park, BJ & Palace, VP 2016, 'Microplastics in aquatic environments: Implications for Canadian ecosystems', Environmental Pollution, vol. 218, pp. 269-80.
Armsworthy, S 1997, Effects of suspended bottom sediment on the feeding activity of the ascidian, Halocynthia pyriformis (Rathke), ProQuest Dissertations Publishing.
Auta, HS, Emenike, CU & Fauziah, SH 2017, 'Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions', Environment International, vol. 102, pp. 165-76.
Bellante, A, Piazzese, D, Cataldo, S, Parisi, MG & Cammarata, M 2016, 'Evaluation and comparison of trace metal accumulation in different tissues of potential bioindicator organisms: Macrobenthic filter feeders Styela plicata, Sabella spallanzanii, and Mytilus galloprovincialis', Environmental Toxicology and Chemistry, vol. 35, no. 12, pp. 3062-70.
Bingham, BL & Walters, LJ 1989, 'Solitary ascidians as predators of invertebrate larvae: evidence from gut analyses and plankton samples', Journal of Experimental Marine Biology and Ecology, vol. 131, no. 2, pp. 147-59.
Brusca, RC, Moore, W & Shuster, SM 2016, Invertebrates / Richard C. Brusca, Wendy Moore, Stephen M. Shuster, Third edition.. edn, Sunderland, Massachusetts U.S.A. Sinauer Associates, Inc., Publishers.
Cole, M, Lindeque, P, Fileman, E, Halsband, C & Galloway, TS 2015, 'The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus', Environmental science & technology, vol. 49, no. 2, p. 1130.
Cole, M, Lindeque, P, Fileman, E, Halsband, C, Goodhead, R, Moger, J & Galloway, TS 2013, 'Microplastic ingestion by zooplankton', Environmental science & technology, vol. 47, no. 12, p. 6646.
Enders, K, Lenz, R, Stedmon, CA & Nielsen, TG 2015, 'Abundance, size and polymer composition of marine microplastics ≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution', Marine Pollution Bulletin, vol. 100, no. 1, pp. 70-81.
Gewert, B, Ogonowski, M, Barth, A & Macleod, M 2017, 'Abundance and composition of near surface microplastics and plastic debris in the Stockholm Archipelago, Baltic Sea', Marine Pollution Bulletin.
Griffiths, MJ, Garcin, C, van Hille, RP & Harrison, STL 2011, 'Interference by pigment in the estimation of microalgal biomass concentration by optical density', Journal of Microbiological Methods, vol. 85, no. 2, pp. 119-23.
Jaffar Ali, HA & Tamilselvi, M 2016, Ascidians in Coastal Water: A Comprehensive Inventory of Ascidian Fauna from the Indian Coast, A Comprehensive Inventory of Ascidian Fauna from the Indian Coast, Springer International Publishing: Cham, Cham.
Klumpp, D 1984, 'Nutritional ecology of the ascidian Pyura stolonifera: influence of body size, food quantity and quality on filter-feeding, respiration, assimilation efficiency and energy balance', Marine Ecology Progress Series, vol. 19, pp. 269-84.
Macginitie, GE 1939, 'The method of feeding of tunicates', The Biological Bulletin, vol. 77, no. 3, pp. 443-7.
Pestarino, M, Fiala‐Medioni, A & Ravera, F 1988, 'Ultrastructure of the branchial wall of a lower chordate: The ascidian Ciona intestinalis', Journal of Morphology, vol. 197, no. 3, pp. 269-76.
Petersen & Svane 2002, 'Filtration rate in seven Scandinavian ascidians: implications of the morphology of the gill sac', International Journal on Life in Oceans and Coastal Waters, vol. 140, no. 2, pp. 397-402.
Petersen, JK, Mayer, S & Knudsen, MÅ 1999, 'Beat frequency of cilia in the branchial basket of the ascidian Ciona intestinalis in relation to temperature and algal cell concentration', International Journal on Life in Oceans and Coastal Waters, vol. 133, no. 2, pp. 185-92.
Petersen, JK & Riisgård, HU 1992, 'Filtration capacity of the ascidian Ciona intestinalis and its grazing impact in a shallow fjord', Marine Ecology Progress Series, vol. 88, no. 1, pp. 9-17.
Seiderer, L & Newell, R 1988, 'Exploitation of phytoplankton as a food resource by the kelp bed ascidian Pyura stolonifera', Marine ecology progress series. Oldendorf, vol. 50, no. 1-2, pp. 107-15.
Young, CM 1988, 'Ascidian cannibalism correlates with larval behavior and adult distribution', Journal of Experimental Marine Biology and Ecology, vol. 117, no. 1, pp. 9-26.
|
|
|
|
|